Clinical Trials
For the general public, clinical trials can be an unfamiliar and misunderstood nuance of the healthcare industry. An understanding of the aims and purposes of a clinical study is an important part of making a decision about whether to participate as a volunteer.
Aims of Clinical Trials
Before healthcare interventions such as medications, devices or psychological therapies are approved to be marketed, they need to pass stringent clinical trial testing. There are three vital criteria for the approval of new medical treatments: whether they work well enough, “efficacy”; whether they are safe enough, “safety”; and whether the body can tolerate them “tolerability”. For a product to become licensed and made available to the public, its benefits in efficacy must be proven to outweigh its risks in safety.SML
Human Research
To identify a molecule with the potential to treat an illness, only around 1 in 5000 compounds studied in the laboratory will meet the criteria to progress to human clinical trials. After positive laboratory results, a compound will spend at least 10 years in human research studies at facilities such as the MAC research clinics, to determine if it is safe and efficacious enough to be licensed for use. Health and ethics authorities are responsible for assessing the risk to benefit ratio of these research studies before they can commence. The research is only given the go-ahead if the proposed trials on the therapy meet their legal and ethical requirements. Subsequently, as a patient or healthy volunteer involved in a MAC study, you will be closely monitored by our experienced medical team and have regular check-ups to ensure your wellbeing.
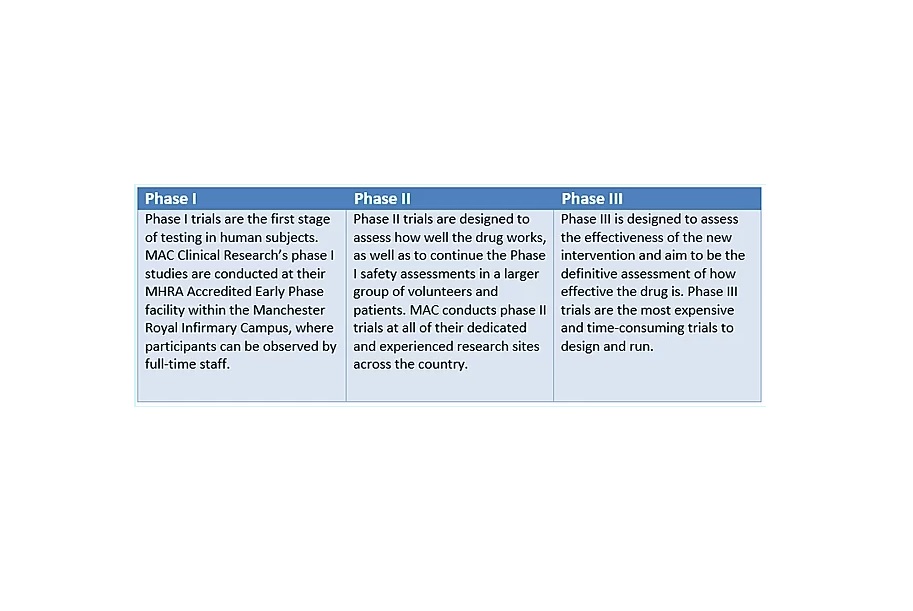
To facilitate safety, clinical trials follow a strict and heavily regulated system of phases, only once a potential treatment passes the previous phase of testing can it proceed to the next.
Data Collected
During the trial, MAC staff will usually collect measurements of vital signs, concentration of the study drug in the blood/tissues, changes to symptoms, and improvement or worsening of the condition targeted by the study drug. This data is then sent electronically to the trial sponsor, who use statistical tests to assess the performance of the medication.
If a drug has proved successful after Phase III trials, the results are passed to the regulatory authorities in different countries for review. If the product is successful, only then will it be granted a licence to be marketed to the public in that country. The MHRA (Medicines and Healthcare Products Regulatory Agency) are responsible for this in the UK.
Speak With Us
At MAC, our recruitment team are always happy to discuss and offer advice relating to our current studies, as well as offering to contact you in the future if any suitable studies arise.Please contact MAC patient recruitment on 0800 633 5507 or you can find a trial here.